The body normally controls the pH of blood within a tight range. One must always remember that pH is a logarithmic scale and so a change from 8 to 7 is a ten-fold increase in H+ concentration. A normal intracellular pH is required for the functioning of many enzyme systems. When blood becomes profoundly acidotic (pH<7) then cellular function becomes impossible and death ensues. There are a lot of texts available describing the causes of the respiratory and metabolic acidosis and alkalosis. However the best way to learn how to interpret blood gases is to practice.
The Advanced Paediatric Life Support Course describes a simple three step system to interpreting paediatric blood gases (do not feel you have to use this method if you already have a good system). You should always keep the Henderson-Hasselbach equation in the back of your mind whilst doing this.
Henderson-Hasselbach equation
CO2 + H2O = H2CO3 = H+ + HCO3-
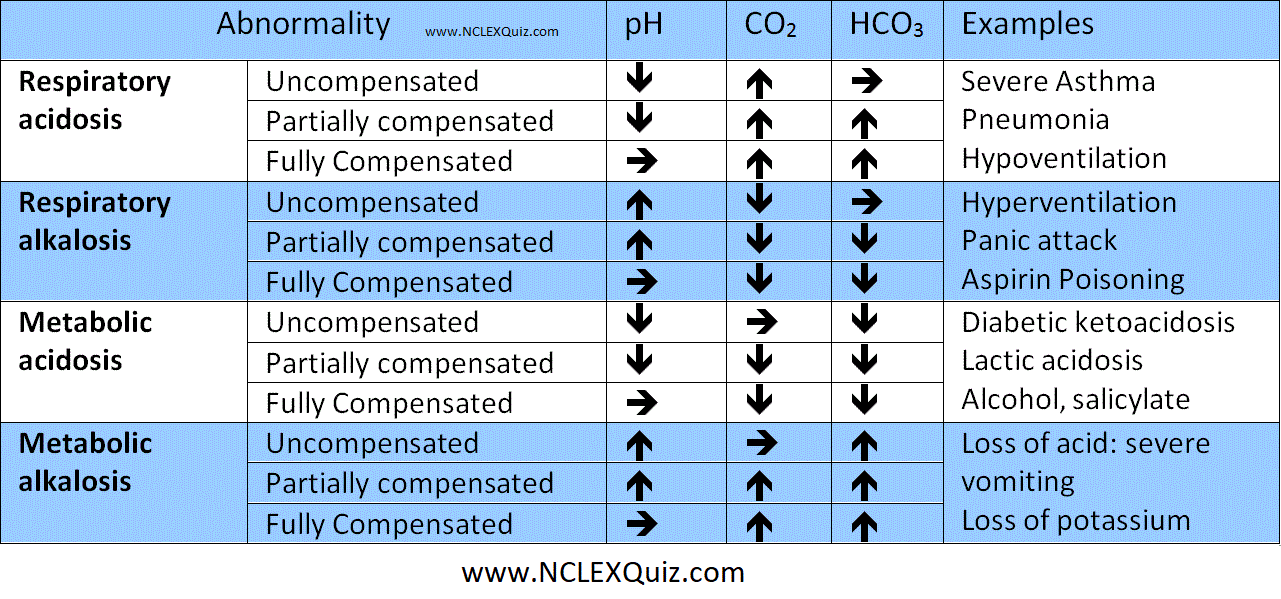
Step 1 – What is the pH? Is there an acidosis or alkalosis?
Step 2 – What is the CO2?
If the CO2 provides a cause of the abnormal pH i.e low pH with a high CO2 (acidosis) or high pH with low CO22 (alkalosis), then the overall picture is RESPIRATORY.
If the CO2 does not provide a cause of the pH, then it is metabolic (possibly compensating).
Step 3 – Confirm your findings by looking at the base excess or bicarbonate.
If the base excess provides a cause for the abnormal pH, i.e. low pH with a negative base excess (acidosis) OR high pH with positive base excess (alkalosis), the overall picture is METABOLIC.
If the base excess does not provide a cause for the pH, it’s compensating for a respiratory abnormality..